

Vitamin K may be administered orally or intravenously. Vitamin K1 (phylloquinone) allows for the synthesis of vitamin K-dependent clotting factors de novo, while fresh frozen plasma (FFP) and prothrombin complex concentrates (PCCs) provide supplemental coagulation factors, including proteins C and S in some preparations. This pathway is less important in coagulation, but it plays a significant role in inflammation and innate immunity. This causes the sequential activation of factors activating the common pathway culminating in fibrin formation. The contact activation pathway is initiated when collagen in the basement membrane of a blood vessel is exposed and a complex of high-molecular-weight kininogen (HMWK), prekallikrein, and FXII is formed. This pathway is more clinically important as it generates the most fibrin in the shortest time. The FVII-TF complex activates the common pathway leading to a large thrombin burst. The TF pathway is activated when an injury to the blood vessel allows factor VII (FVII) to come in contact with TF, which is expressed on stromal fibroblasts and leukocytes. Two pathways exist to initiate the cascade: the tissue factor (TF) pathway (formerly called the extrinsic pathway) and the contact activation pathway (formerly the intrinsic pathway) ( Figure 1). This involves the generation of fibrin as a result of activation of the clotting cascade. Mechanical methods of hemostasis may be necessary, including direct compression, surgery, or embolization. Providers should remember that all patients with emergent or life-threatening bleeding require attention to basic interventions, including cessation of anticoagulation therapy, blood product transfusions, and assessment for airway protection. We will review physiologic hemostasis processes, the effect of anticoagulation on normal hemostasis, and then discuss each anticoagulant and its reversal. 3 Owing to the propensity of anticoagulated patients to bleed, a strategy for reversal of anticoagulation induced by any of the common agents is essential for the treating clinician.
Compared to warfarin, these drugs have generally been associated with lower rates of major hemorrhage and a reduction in the risk of fatal bleeding and intracranial hemorrhage (ICH). In addition, the use of the direct oral anticoagulants (DOACs), such as factor Xa and factor IIa (thrombin) inhibitors, is rapidly increasing.

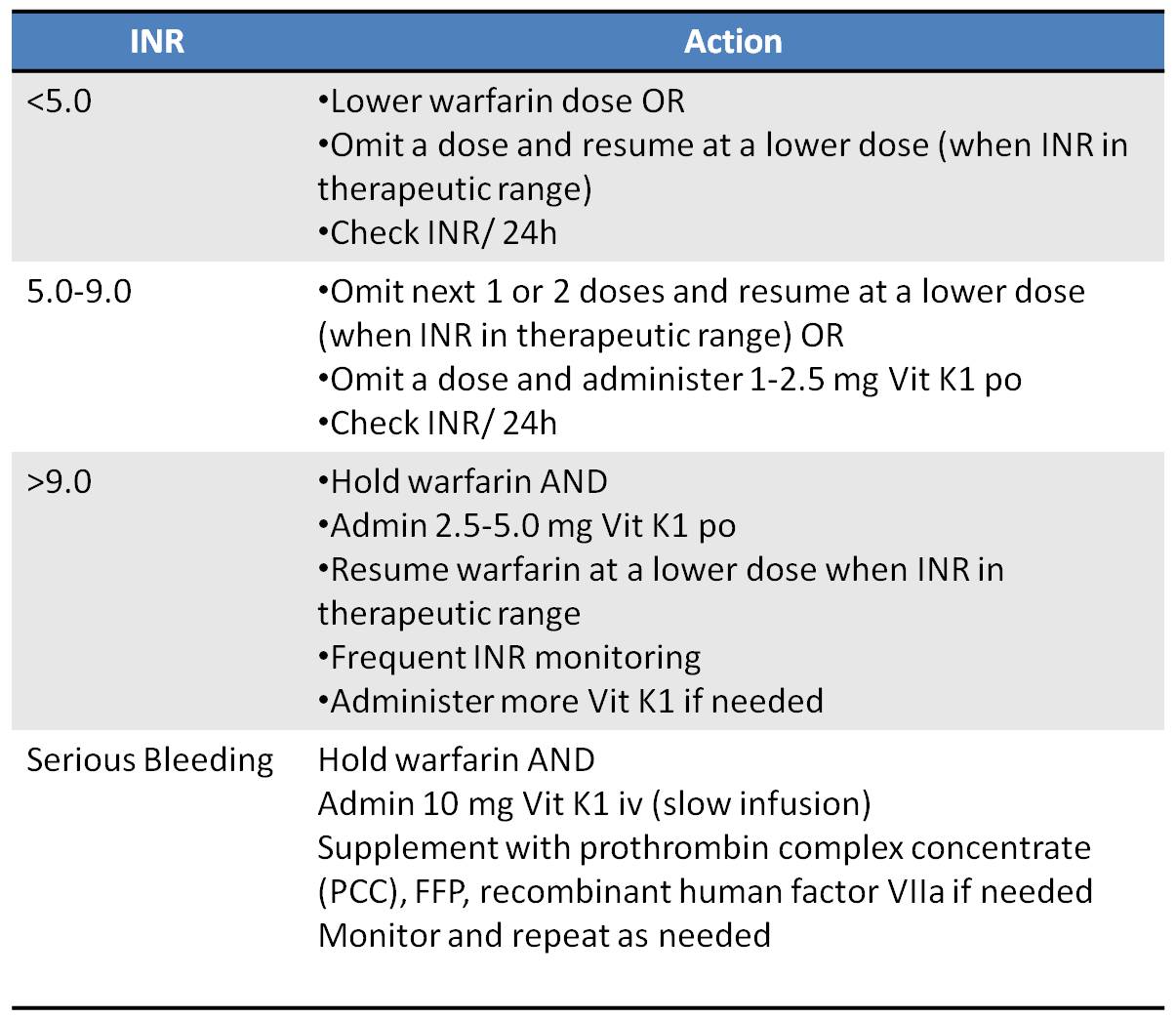
2 This does not include the many additional disease processes for which warfarin was indicated. 2 In 2010, atrial fibrillation alone prompted about 30 million prescriptions for warfarin. 1 The incidence of bleeding while on warfarin has been estimated at 15–20% per year, with life-threatening bleeding occurring at a rate of 1–3% per year. The risk of bleeding varies with the type of anticoagulant agent used. Inappropriate bleeding is the most concerning complication of anticoagulant therapy.


 0 kommentar(er)
0 kommentar(er)
